Dr. Rana Choudhary
(MBBS, DNB, DGO, DFP, DCR, FCPS, FICMCH, FICOG, MNAMS)
Masters in Reproductive Medicine & IVF (UK)
Consultant Obstetrician & Gynaecologist
Reproductive Medicine (Fertility) Specialist
Certified Personal Counsellor
Introduction
IUI is a procedure to treat infertility in which washed and prepared sperms are introduced into the woman’s uterus, with timed ovulation, with the aim of getting the maximum number and most motile sperms nearer to the ovum and achieving pregnancy. The history of intrauterine insemination (IUI) goes back to the 18th century when Scottish surgeon John Hunter performed an effective procedure with the use of the husband’s sperm. In 1884, after William Pankhurst from Philadelphia in the United States applied the donor’s sperm, live birth was reported. ESHRE data indicate that the pregnancy rate per cycle (PR per cycle) after IUI-H is 12.4% compared with 28.9% after IVF and the results have not changed over the last few years.
History taking:
Various important point during history taking are summarized in the table below
Table 1: Important points to be considered during history taking of a couple presenting with infertility
| Sr. No. | Factor | Specific findings/ questions | Importance |
| 1 | Name | For identification of the patient | Better rapport |
| 2 | Age, Duration of infertility | To decide the approach | More the age and duration of infertility, more aggressive is the approach as chances of conception goes on decreasing |
| 3 | Occupation | Shift duties etc | Decrease in frequency of intercourse |
| 4 | Sexual history | Vaginusmus, Sexual satisfaction etc | Decrease in frequency of intercourse |
| 5 | Obstetric history | H/o previous abortions, RPL, D & C | Endometrium may be defective |
| 6 | Medical history | h/o DM, HT, Kochs, Thyroid problems | Pregnancy may be high risk |
| 7 | Surgical history | H/o surgeries in pelvis and abdomen | Adhesions leading to altered tubo-ovarian anatomy |
Other aspects :
- Both partners should be seen together
- Privacy & sufficient clinical time
Positive predictive factors for IUI success
- Age of female partner < 35 years {should we consider a lower age (33years) for Indian women as their ovarian reserve gets depleted earlier?}
- Duration of infertility < 5 years
- At least one functional normal fallopian tube and no uterine factor leading to infertility
- Adequate ovarian reserve (based on Sr. AMH, Antral follicular count, Day 2 FSH)
- Good semen parameters
Variables affecting IUI success
Treatment with ovulation induction and IUI is more successful in an-ovulatory women. Various factors affect the success rate of IUI.
- Age of female -As age advances the chances of pregnancy after ovulation induction and assisted reproductive procedures (ART) procedures like IUI decreases.
- Unexplained Infertility – Addition of IUI to super ovulation produces better results than super ovulation and timed intercourse (11.37%) especially in cases of unexplained infertility.
- Endometriosis – Mild or minimal endometriosis may benefit from IUI (pregnancy rates 7% to 18%), however in severe cases it is not advisable (4.5%).
- Cervical Factor – Conception rate in natural cycle is 14% and in IUI plus Gonadotrophins is 17%.
- Male factor infertility –Total motile sperm count (TMSC) >5 million/ml and sperm morphology are most valuable sperm parameters to predict IUI outcome.
When To Start Investigations & Treatment?
Table 2: When to start investigations in of a couple presenting with infertility
| Sr. No. | Parameters | When to start investigation |
| 1 | <35 Years | 1 Year |
| 2 | >35 Years | 6 Months |
| 3 | 40 Years & above | At the earliest |
| 4 | Oligomenorrhoea | At the earliest |
| 5 | Amenorrhoea | At the earliest |
| 6 | Abnormal pelvic ultrasonography | At the earliest |
| 7 | History suggestive of ↓ ovarian reserve | At the earliest |
| 8 | H/o previous surgeries eg salpingectomy or disease such as Kochs abdomen | At the earliest |
Ideal Body Weight (IBW)
Studies have shown that increased body weight / obesity interferes with ovulation & causes infertility
Calculate IBW using Brocas index
Males: (wt in kg) = Height (in cm) – 100
Females: (wt in kg) = Height (in cm) – 105
Table 4: Classification of obesity
| Sr. No | Body weight | Type of obesity |
| 1 | 20% > Ideal body weight (IBW) | Obese |
| 2 | Body weight > 200 lbs | Morbidly obese |
| 3 | > 2 x Ideal body weight (IBW) | Morbidly obese |
Table 5: Evaluation of Female Partner for infertility
| Sr. No. | Evaluation of | Test | Importance |
| 1 | Ovulatory function | TSH, Prolactin, AMH, TVS | Distinguish between ovulatory & non ovulatory women AMH – To know ovarian reserve |
| 2 | Pelvic Structure | Detailed transvaginal ultrasound | Detect structural abnormalities like poly, fibroid, cysts etc |
| 3 | Tubal patency testing | HSG/ Laparoscopy / Hysteroscopy | Extremely important before IUI |
Flowchart 1: Management of thyroid abnormalities
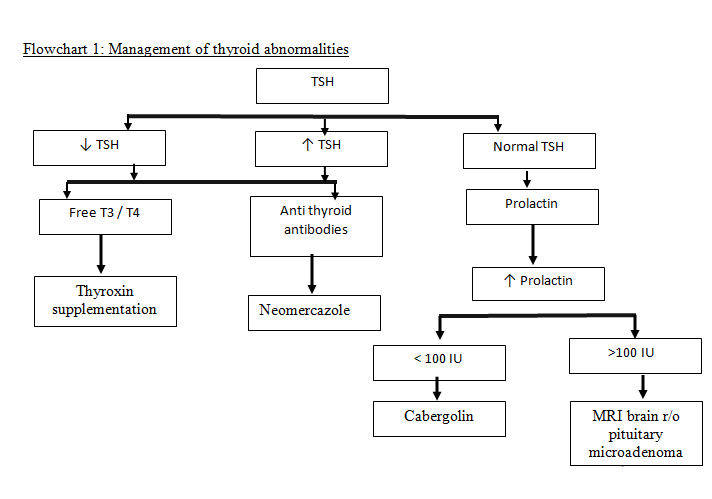
Direct beneficial effect of TSH on follicular recruitment warrants investigation of serum thyroid levels and thyroxin supplementation in infertile women with Sr. TSH ≥2.5 units in attempts to improve recruitment.
Table 6: Insulin resistance and infertility
| Insulin resistance (IR) | |
| Measurement of Insulin resistance | Fasting glucose (mmol/L) ÷ Fasting Insulin (micro IU/ml) |
| IR Ratio < 4.5 | Indicates Insulin resistance (IR) |
| For eg. FBS = 100 & Fasting Insulin is 45 Ratio = 100 ÷ 45 = 2.22 | Indicates Increased Insulin Resistance |
Anti Mullerian Hormone (AMH) test :
- Produced by Preantral & small antral stage (< 4 mm)
- No AMH is secreted by follicles > 8 mm
- Can be measured on any day of cycle but better on day 2/3
- Measurement of AMH = Biological Body Clock Test
- Predicting live birth outcome, ovarian response & OHSS
- May reflect fertility potential more accurately than conventional markers like FSH, inhibin B or estradiol levels
- Should be done from a GOOD STANDARDISED LABORATORY as lab to lab variation is marked !
- Relatively poor predictor for Pregnancy outcomes
- Low AMH levels in isolation do not represent an appropriate marker for withholding treatment
Table 7: Importance of Anti Mullerian Hormone (AMH) test
| Sr. No. | Sr. AMH levels | Significance |
| 1 | < 0.3 ng/ml | Few eggs remaining |
| 2 | >2.5 ng/mL | Probably normal reserve |
| 3 | > 3.6 ng/mL | Increased risk OHSS |
Ovarian reserve markers / tests
Age related decline in female fertility well recognised. It starts at 30 years with rapid decline after 37 years. Commonly used ovarian reserve tests are :
D2 antral follicle count (<5 ,Poor outcome)
- AMH of 2 to 6 (<1 Poor ovarian reserve, >6 PCO)
- D2 FSH > 10 IU/l poor response to ART
- No evidence for ovarian volume, ovarian blood flow, inhibin B, estradiol (E2)
Table 8: Possible factors suggestive of Poor Ovarian Reserve OR Pre mature ovarian aging
- Short follicular phase
- Past h/o of surgeries – Endometriosis, ovarian cysts, adhesions
- AFC less
- Low AMH
- P/h/o poor response to treatment
Table 9: Investigations to evaluate tubal patency
| Sr. No. | Investigation | Advantages | Disadvantages |
| 1 | HSG | Screening test | Not very specific |
| Ideal cost effective | False positivity rate is high | ||
| Non-invasive | |||
| Pre-operative help in counseling | |||
| Laparoscopy | Gold standard | Cost | |
| High specificity & sensitivity | Invasive |
Conclusion:
- Adequate & Compassionate Counseling
- Do not bombard with investigations as it further increases stress in patients
- Each patient is different, hence blanket treatment is not justified
- Keep a watch on couples age …especially ovarian aging as chances of pregnancy decreases exponentially, as age of the female partner advances
- Give patients an idea about real expectations/ results so that they can prepare themselves
- Timely referral
- Individualization is the key to success
Male Factor Evaluation
- Detailed history – Exposure to high temperature ,environmental toxins etc
- Thorough clinical evaluation – esp in relation to any local genital / scrotal swelling eg. Varicocoele, Hydrocoele etc
- Lab investigations
Fig 1 : Adverse Effects Of Obesity on Semen parameters
| Excess peripheral adipose tissue (High aromatase activity) Testosterone → Estradiol Androstenedione → Estrone Excess of suprapubic & inner thigh fat scrotal temperatures All these can cause ↓sperm count as well as motility |
Table 10: Physical Examination in males with infertility
| Sr No. | Factor | Importance |
| 1 | General examination Stature, Height ,Weight , BMI, BP | |
| 2 | Increased BMI | R/o DM |
| 3 | TPR / BP | R/o HT |
| 4 | Gynecomastia | Hormonal imbalance |
| 5 | Hair distribution & amount | Subvirilization |
| 6 | Genital – Spermatic cord, Scrotum, Testis (mobility, consistency), Abnormal shapes of penis, urethral meatus | Only in indicated cases with abnormal semen analysis |
| 7 | Azoospermia | Rectal exam to exclude ejaculatory duct obstruction |
Table 11: Investigation of Male Partner for infertility
| Sr No. | Factor | Importance |
| 1 | Detailed Semen Analysis- Count, Motility and Morphology | Treatment depends primarily on this report |
| 2 | Viral markers – HBsAg , HIV, HCV | Required for IUI |
| 3 | Semen culture | If semen analysis shows leucospermia |
| 4 | Sperm Function Test | If semen analysis shows moderate to severe Oligoasthenozooteratospermia (OATS) |
| 5 | Sr. FSH | If semen analysis shows moderate to severe Oligoasthenozooteratospermia (OATS) To differentiate cause of AZOOSPERMIA – Obstructive versus Non Obstructive Low in hypogonadotrophic hypogonadism |
| 6 | Sr. LH | Low in hypogonadotrophic hypogonadism |
| 7 | Sr. Testosterone | If semen analysis shows moderate to severe Oligoasthenozooteratospermia (OATS) |
| 8 | Sr Prolactin | Erectile dysfunction |
| 9 | Blood Sugar | DM |
| 10 | Colour Doppler | Clincial examination s/o Varicocoele If semen analysis shows moderate to severe Oligoasthenozooteratospermia (OATS) |
| 11 | Karyotype | If semen analysis shows moderate to severe Oligoasthenozooteratospermia (OATS) |
| 12 | Y chromosome Microdeletion | If semen analysis shows moderate to severe Oligoasthenozooteratospermia (OATS) H/o repeated implantation failure / abortions in first trimester |
Semen Analysis
- Cornerstone’ of lab evaluation (although it is not a sperm function test)
- Gives information – Functional status of seminiferous tubules, epididymides & accessory sexual glands
- Prostatic gland fluid (0.5ml – zinc, citric acid, acid phosphatase & proteases)- assures liquefaction
- Seminal vesicle fluid (1.5-2ml -prostaglandins & fructose)
- Semen sample must be collected after min 3 days & max 7 days of abstinence
- Semen analysis – In Clinical Practice, male factor infertility is identified by ABNORMAL SEMEN PARAMETERS
- Interpretation of semen analysis report
- Only from a standardised laboratory
- Any abnormal report must be confirmed by at least two reports, atleast 4 weeks apart
- Judge response to treatments after 3 months of therapy as one spermatogenic cycle (testicular) lasts from 63 – 72 days
Table 12: WHO (2010) guidelines for normal semen reporting
| Semen parameters | Normal value |
| Semen volume | 1.5 ml |
| Total sperm in the ejaculate | 39 million |
| Sperm per ml | 15 million/ml |
| Vitality | 58% live |
| Progressive motility | 32 % |
| Total motility | 40 % |
| Morphologically normal | 4 % |
Ref: World Health Organization. NICE guideline CG156, recommendation 1.3.1.1. Oct 2014
| Success of IUI – Total Motile Sperm Count (TMC) & Morphology TMSC = Count x Volume x % Motility eg (10 million x 4 ml x 50) ÷ 100 = TMC 20 million |
Table 13: Semen parameters and treatment required
| Sr. No. | Semen parameters | Treatment required |
| 1 | 5 Million (Pre wash count) | IUI |
| 2 | 1 million (Postwash) | IUI |
| 3 | Pre wash 1-5 million | IVF |
| 4 | Pre wash <1 million | ICSI |
- Success in IUI is highest with ≥ 14 % sperms with normal morphology, Intermediate with 4 – 14 % and poor with < 4 %
| Poor sperm count doesn’t rule out any pregnancy possibility Normal count doesn’t guarantee fertilization/pregnancy |
Table 14: Sperm Function Tests
| Sr. No. | Test | Importance |
| 1 | Hypo-osmotic swelling (HOS) test | Integrity & behavior of the cell membrane of the sperm tail |
| Helps to distinguish between immotile alive & dead sperms | ||
| 2 | Sperm DNA Fragmentation Test | Sperm quality is dependent on the amount of damage to the sperm DNA or DNA fragmentation |
Indication for Sperm Function Tests –
- All men with abnormal semen analysis
- Advanced age
- Infection
- Normal semen analysis but failed IVF for unexplained reasons
- May help predict success of a procedure
Table 15: Management tips during evaluation for Varicoele
| Sr. no. | Test | Importance | Management |
| 1 | Grade 1 & 2 Varicoele | No surgery is required | A trail of medical therapy can be given, followed by IUI |
| 2 | Grade 3 | Surgical management | Surgery |
| 3 | Positive Predictive factors of varicocelectomy in grade 3 varicocoele | Lack of testicular atrophy Normal FSH Total sperm count more than 5 million |
Table 16: Golden Rules For Investigations
| Clinically relevant and effectiveShould impact the line of treatmentEasy to interpretFeasible – cost, convenienceInvestigations are seldom more important than the treatment or the res |
References:
- Diagnostic evaluation of infertile female: a committee opinion. The Practice Committee of the American Society for Reproductive Medicine. Fertility & Sterility, Aug 2012.
- What affects functional ovarian reserve, thyroid function or thyroid autoimmunity? Reprod Biol Endocrinol. 2016 May.
- Med Pregl. 2016 Jan-Feb;69(1-2):25-30.
- What affects functional ovarian reserve, thyroid function or thyroid autoimmunity? Reprod Biol Endocrinol. 2016 May
- Evaluating the performance of serum antimullerian hormone concentration in predicting the live birth rate of controlled ovarian stimulation and intrauterine insemination. Fertil Steril. 2010 Nov
- World Health Organization. NICE guideline CG156, recommendation 1.3.1.1. Oct 2014
